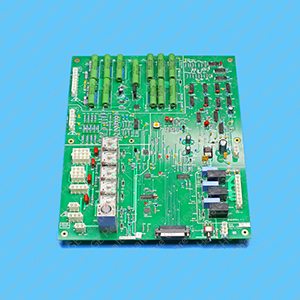
NGPDU Control Board 2334820-3
| 2334820-3 | |
| 2334820-2 2334820 2334820-30 | |
| New | |
| Computed Tomography (CT) | |
| GE Healthcare | |
| GE HealthCare | |
| N/A | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Has good dimensional stability
- Packaging adheres to global specifications and is free of any physical damage
- Low environment impact
- Enhanced durability and reliability
Frequently purchased with this item
Product Overview
The NGPDU Control Board used on MAC 3500 in Computed Tomography (CT) is used in Optima 660 and other medical equipments as applicable. The NGPDU Control Board located within the PDU will provide the sequencing of the various sub-system power contactors as commanded by the system. It is a circuit board which includes resistors, capacitors, connectors, diodes, IC’s, LEDs, inductors and switch. The product is made from the material which possess corrosion resistant, impact resistant, ductility, excellent performance and increased lifespan. The circuit board is a ROHS compliant and is approved for today’s safety standards. They are durable and compact with a good dimensional stability. It is free from dust, burrs, sharp edges, surface blemishes, sink marks and flash. The GE product is an innovation and technology, which fits well into versatile customer needs. The product is securely packaged inside a high quality packing box to avoid physical damage during transit and labeled with details about the product, Quality Assurance (QA) seal and shipment details.
Compatible Products

Optima 660

Optima 540

VCT

Revolution EVO

Revolution CT

Lightspeed RT Xtra

Lightspeed RT

Lightspeed Pro 16

Discovery 750 HD

Brightspeed Edge

Lightspeed 16

Lightspeed Ultra

HiSpeed Qxi

Brightspeed Excel

Brightspeed Elite

Lightspeed Plus

Lightspeed QXi

Discovery 670

Discovery 670CTZ

Discovery 670 Pro

Discovery ST

Discovery ST (4 Slice)

Discovery ST (8 Slice)

Discovery ST (16 Slice)

Discovery ST (64 Slice)

Discovery ST (128 Slice)

Discovery STE (8 Slice)

Discovery STE (16 Slice)

Brivo 385

Optima 520
Equivalent Item(s):
Below is more information on the equivalent item(s). Items without a hyperlink are listed for reference only and are not available for purchase online.
| Equivalent Item(s) | Item Details |
|---|---|
| 2334820-2 | 2334820-2 |
| 2334820 | CTL BOARD IN NGPDU |
| 2334820-30 |
Return And Exchange
GE HealthCare will accept, for return from customer, items that are in new condition, unworn, unaltered and free of damage. Items cannot be returned or exchanged beyond 30 days from customer’s receipt of the original order and all returned items will be subject to a 15% restocking fee. To obtain a full refund or exchange of the item within the 30 day return period (subject to the 15% restocking fee) customer must request a return materials authorization (RMA) from GE HealthCare and return the item to GE HealthCare utilizing this RMA number. Items must be returned in the original packaging, unused and free of damage.
Please note, cuffs, BP items, sterile and hazmat items cannot be returned. If a replacement order is requested, the restocking fee may be waived at GE HealthCare’s sole discretion.